Date: 1-MAY-2020 Last Updated: 2-FEBRUARY-2026
Rapid Adsorption in Autosampler Vials: A Critical but Overlooked Source of Quantitative Error
Autosampler vials are often treated as passive containers, but for many analytes—especially basic, cationic, or surface‑active compounds—they can become an unexpected source of data variability. Adsorption to borosilicate glass surfaces is time‑dependent, yet can occur within minutes, significantly altering analyte concentration before the first injection sequence is completed.
MICROSOLV's internal experiments highlight how vial surface chemistry—not just instrument conditions—can directly affect quantitation, method robustness, and reproducibility.
1. Adsorption to Standard Glass Autosampler Vials Happens Faster Than Many Labs Realize
Borosilicate glass contains silanol groups that interact strongly with basic analytes. These interactions cause partial or significant loss of analyte concentration, manifesting as diminished peak areas. This process accelerates in low‑concentration solutions, making trace‑level assays particularly vulnerable.
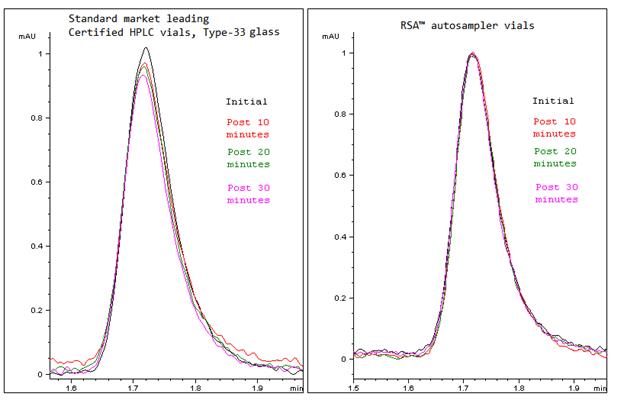
In controlled experiments using 5 ppm cetylpyridinium chloride, peak area in a standard Type‑33 borosilicate “certified” vial declined markedly in just 15 minutes after preparation. See chromatograms below.
Even during short autosampler queue times, this time‑dependent adsorption can alter:
- Reported concentration
- Relative response factors
- System suitability metrics
- Mass percentage or stability study outcomes
For regulated workflows, the risk is especially consequential.
2. RSA™ (Reduced Surface Activity) Vials Preserve Analyte Integrity
MICROSOLV’s RSA™ vials are engineered to eliminate the reactive silanol sites that drive adsorption of basic molecules. These vials provide a drastically more inert surface—an effect clearly shown in chromatographic comparisons.
In the same experiment, RSA™ vials showed minimal or no change in peak area over the same time interval, preserving analyte concentration during typical autosampler dwell times.
Key advantages of RSA™ vials:
- Greatly reduced adsorption of basic or cationic analytes
- Improved repeatability across injections
- Stable peak area during batch sequences
- Lower risk of false degradation trends in stability studies
3. Why This Matters in Modern LC, LC‑MS, and HPLC Workflows
As analytical methods become more sensitive, particularly in LC‑MS/MS:
- Lower sample concentrations increase adsorption susceptibility
- Batch runs can span hours, increasing exposure time
- Autosampler wait times (queue delays) amplify concentration drift
- Regulatory and quality systems require tighter performance windows
Neglecting vial surface activity can result in:
- Failing system suitability
- Unexplained low recoveries
- Poor linearity at low concentrations
- High variability in bioanalytical assays
- Apparent “instability” falsely attributed to sample degradation
4. Best Practices for Technical Users
✔ Select vial chemistry based on analyte properties
Basic, cationic, peptide‑like, or surfactant‑type analytes should not be run in untreated glass.
✔ Use RSA™ vials for low‑concentration or adsorption‑prone analytes
These provide the most inert glass option currently available for HPLC/LC‑MS workflows.
✔ Reduce dwell time before injection
Prepare samples close to run time when possible—particularly for sensitive analytes.
✔ Evaluate vial performance during method development
Include timed re‑injections to check for adsorption‑related peak decay.
5. Summary for Technical Chromatographers
Adsorption onto standard borosilicate autosampler vial surfaces is rapid, measurable, and capable of significantly altering results—even within minutes. For analytes prone to surface interactions, RSA™ vials offer a validated, surface‑inert solution that preserves sample integrity and ensures reliable quantitation throughout the entire autosampler sequence.
In the chromatograms below, you can observe significant peak area diminishing in the standard HPLC vials (left), in just a matter of fifteen minutes from the immediate prep and analysis, versus the peak area in RSA™ Vials (Right).